Effects of heme precursors on CYP1A2 and POR expression in the baculovirus/Spodoptera frugiperda system☆
Huiyuan Lu, Jun Ma, Nian Liu, Shoulin Wang
Key Lab of Modern Toxicology of Ministry of Education, School of Public Health, Nanjing Medical University, Nanjing 210029,Jiangsu Province, China
INTRODUCTION
Cytochrome P450 (CYP450)monooxgenases, make up 70%-80% of all phaseⅠxenobiotic-metabolizing enzymes. They can metabolize a large number of endogenous compounds and are involve in many cellular functions[1]. The CYP450s superfamily consists of many subfamilies, including CYP1A, 2A, 3A,1B, 1C, and so on. CYP1A2 belongs to the CYP1A subfamily, which constitutes approximately 13% of the total liver CYP content[2]. CYP1A2 is involved in the metabolism of several endogenous compounds and widely used drugs, and in the activation of procarcinogens such as aflatoxin B1, the commonly recognized hepatocarcinogen[3-4]. Since CYP450s are a group of biocatalysts, sources of electrons are necessary for their activation. The addition of two electrons (reduction)to the heme iron of CYP450s may break the hard oxygen-oxygen bond. NADPH-CYP450 oxidoreductase (POR)is a single protein which transfers electrons from NADPH to CYP450s in the endoplasmic reticulum[5-6]. It is usually regarded as the partner of the CYP450s in the metabolism of xenobiotics and endogenous compounds.
Although the primary structures of CYP450 enzymes are well known, studies on functional properties of different eukaryotic CYP450s have lagged behind because of the lack of efficient systems for heterologous expression of catalytically active enzymes[7]. A number of systems, including both prokaryotic (e.g., E.coli)and eukaryotic (e.g., yeast,insect cells, and mammalian cells)systems have been used to express mammalian CYP450s. Among these,the Bac-to-Bac baculovirus expression system usually yields the highest level of expression of CYP450s because the baculovirus shuttle vector in this system has an integrity genome of Autographa californica multiple nuclear polyhedrosis virus (AcMNPV, Ac)and can increase the expression efficiency through site-specific transposition[8]. In addition, Spodoptera frugiperda (sf9)insect cells are very important for the efficient expression of CYP450 because they have similar modes for post-transcription and posttranslation of proteins to mammalian cells. As a result, the baculovirus/sf9 expression system can yield high levels of unmodified, native CYP450 proteins,except for the noted deficiency of sf9 cells in heme incorporation[8]. Thus the addition of a heme precursor is necessary to compensate the baculovirus/sf9 system in order to maximize the expression of the functional CYP450s.
CYP450s is a heme-containing protein, so the heme iron in CYP450s plays a very important role in their catalytic activations. Heme refers to the ferrous iron(Fe2+)bound to tetrapyrrole, a macrocyclic porphyrin,and its oxidized form (Fe3+)is known as hemin. Hemin can provide heme to synthesize CYP proteins in the baculovirus/sf9 system. δ-Aminolaevulinic Acid (5-ALA)is the first committed intermediate of the heme biosynthesis pathway in vivo, and ferric citrate can provide ferrous iron for this pathway as well as heme for the baculovirus/sf9 system. POR should be taken into consideration for P450s heterologous expression because POR content is rate-limiting for CYP reactions[9]. Previous studies showed that functional CYP450s could be obtained by adding cofactors(e.g., 5-ALA[10], ferric citrate, or hemin[11,12]), and the approach of POR and CYP450s co-expression via coinfection enabled the production of enzymatically active CYP450s in the natural microsomal membrane[10]. These methods provide possible ways to reconstitute catalytically active systems in vitro.Because of the complication of CYP450s expression in vitro, systematic studies should be undertaken to find the most favorable conditions for stable and efficient expression of CYP450s.
In the current study, the baculovirus/sf9 system was utilized for the expression of CYP450s, POR, and their co-expression. The influences of different cofactors such as hemin, 5-ALA and Fe3+were considered to optimize the expression system. This would help to provide a reliable research model for constructing the metabolism of xenobiotics in vitro.
MATERIALS AND METHODS
Reagents
Ferric citrate, 5-ALA, cytochrome c, and a monoclonal anti-CYP1A2 antibody were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sf9 insect cells, Bac-to-Bac baculovirus expression system,Cellfectin? reagent, unsupplemented Grace Insect Cell Culture Medium and SF900 Ⅱ SFM were obtained from Invitrogen (Carlsbad, CA, USA). Rabbit antihuman POR polyclonal antibody was from Millipore(Bedford, MA, USA). Anti-mouse IgG, horseradish peroxidase-linked whole antibody (from sheep), and the enhanced chemiluminescence (ECL)western blot detection reagents were purchased from Cell Signaling Technology (Danvers, MA, USA). Emulgen 911 was obtained from Karlan Research Products Corporation(Cottonwood, AZ, USA). Solid sodium dithionite was purchased from Sinopharm (Shanghai, China).
Expression of human CYP1A2 and POR proteins in the baculovirus/Sf9 system
All the proteins were expressed in the Bac-to-Bac baculovirus expression system (Invitrogen, Carlsbad,CA, USA)following the manufacturer's instructions.Briefly, the CYP1A2 and POR cDNAs were first cloned into a pFastBac?1 vector to construct the donor vectors,and then were transformed into MAX Efficiency?DH10Bac? chemically competent cells (Invitrogen,Carlsbad, CA, USA)to form the recombinant bacmid which were subsequently used to transfect the sf9 cells to produce recombinant baculovirus particles, and further to get the baculoviral stock via infecting the sf9 cells. Ferric citrate (0.1 M in double distilled water)and/or 5-ALA(0.1 M in SF900 ⅡSFM medium), or hemin (2 mg/ml in 50% ethanol and 0.2 M NaOH)was then added to the culture medium. After 72h-incubation, the infected sf9 cells were harvested, washed twice with 0.1 M potassium phosphate buffer (pH 7.4)and re-suspended in the reaction buffer [0.1 M potassium phosphate containing 20% (V/V)glycerol, 1 mM fresh PMSF, 0.1 mM EDTA, 0.1 mM DTT, 0.5%(V/V)Emulgen 911].The microsomes were prepared by sonicating with an Ultrasonic Processer (CPX130, Cole-Parmer,USA)and centrifuging as described elsewhere[13]. The prepared microsomes were stored at -80°C before use.
P450 content determination
The P450 content was determined by reduced CO-differential spectra following the previous study[14].Microsomal proteins were diluted to 1 mg/ml with the reaction buffer. The protein solution was placed in a 1 cm optical path cuvette and reduced by a few milligrams of solid sodium dithionite. After the baseline was recorded, the sample cuvette was bubbled with CO for 60 sec. The difference in spectrum absorption was measured by scanning from 550 nm to 400 nm using an UV/visible spectrophotometer (DU?800, Beckman coulter, USA).
POR activity determination
Cytochrome c assay was used to determine the activity of POR. In brief, 840 μl reductase assay buffer (containing 0.05 M potassium phosphate, 0.1 mM EDTA, 0.3 M KCl,pH7.4)was put into a 1cm optical path cuvette, and then about 20 μl microsomal protein (dilute about 30 times)and 100 μl 0.8mM cytochrome c were added. After the base-line was recorded, 50 μl 4 mM NADPH was added and mixed immediately to start the reaction.The spectrophotometric rate was measured at 550 nm.
Immunoblot analysis
Immunoblotting for measuring CYP1A2 and POR protein levels was carried out as described previously[15]. Microsomal proteins were separated by SDS-polyacrylamide gel electrophoresis, transferred to a nitrocellulose sheet, and then incubated with a monoclonal antibody against human CYP1A2 as the primary antibody (1:5000), followed by the binding of a secondary antibody (goat anti-mouse IgG, 1:2000)conjugated with horseradish peroxidase. In order to detect POR, a polyclonal antibody against human POR was used as the primary antibody (1:2000)and an anti-rabbit IgG conjugated with horseradish peroxidase as the secondary antibody (1:2000). The immunoblots were visualized using ECL detection according to the manufacturer's protocol. For the densitometric analysis, the protein bands on the blot were measured using BandScan 4.3 software.
Statistical analysis
Data are expressed as mean±SD. Statistical differences were analyzed using one-way analysis of variance (ANOVA), and the LSD method was used for multiple comparisons. P-values less than 0.05 were considered statistically significant.
RESULTS
Construction of recombinant pFastBac?1-CYPs (POR)and Ac-Bacmid-CYPs (POR)
Recombinant plasmids of pFastBac?1-CYPs(POR)were obtained via double digestion by restriction enzymes and Quik T4 DNA ligase and verified by restriction endonuclease analysis. The recombinant plasmid pFastBac?1-CYP1A2 digested with KpnⅠ/XbaⅠgenerated a 1.5 kb fragment (total length of CYP1A2 gene is about 1.5 kb, GeneBank No.: NM_000761)and a 4.7 kb fragment (equal to the length of vector, Fig.1A), while pFastBac?1-POR digested with BamHⅠ/ KpnⅠgenerated a 2.0 kb fragment (total length of POR gene is about 2.0 kb, GeneBank No.: NM_000941)and a 4.7 kb fragment (Fig.1B). Reconstructed Ac-Bacmid-CYPs(POR)were obtained by transforming pFastBac?1-CYPs(POR)into the bacmid of competent DH10Bac cells and verified by PCR. The results showed that Ac-Bacmid-CYP1A2 generated a 1.5 kb fragment (using primers of 1A2 Forward: 5'-ATGGCATTGTCCCAGTCT GTTCC-3' and 1A2 Reverse: 5'- TCAATTGATGGAGAAGCGCCGCGCCTG-3', Fig.1C), and Ac-Bacmid-POR generated a 4.3 kb fragment (using primers of M13 Forward: 5'-GTTTTCCCAGTCACGAC-3' and M13 Reverse: 5'-CAGGAAACAGCTATGAC-3',Fig.1D). Finally, the reconstructed Ac-Bacmid-CYPs (POR)were transfected into sf9 cells to obtain recombinant baculovirus particles.
Heterologous expression of human CYP1A2 and POR in baculovirus/sf9system
Sf9 cells were infected by CYP1A2 or POR recombinant baculovirus particles, and then the traditional conditions were used to express these proteins: ferric citrate and 5-ALA stock solutions were added to cell culture medium with a final concentration of 0.1 mM. Infected cells were harvested after an incubation time of 72h. Immunoblot analysis detected a single protein band with the expected molecular weight (55KD and 78KD respectively)(Fig.2A and 2C). In the CO-difference spectroscopy analysis,CYP1A2 protein displayed a characteristic absorption peak at 450 nm (Fig.2B)and the content was 0.21 nmol/mg protein. Cytochrome c assay revealed that POR activity was 1,519.47 unit/mg protein.
Effects of different factors on CYP450s (POR)expression
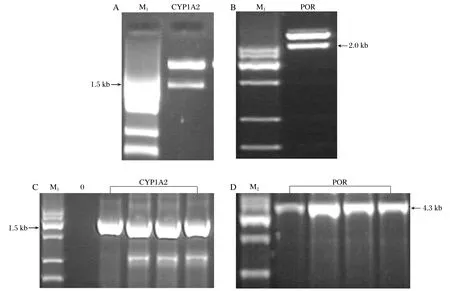
Fig. 1 Identification of the recombinant transposing plamid by restriction endonulease digestion and PCR. M1:MarkerⅦ. M2: MarkerⅣ.A and C: CYP1A2 gene. B and D: POR gene.
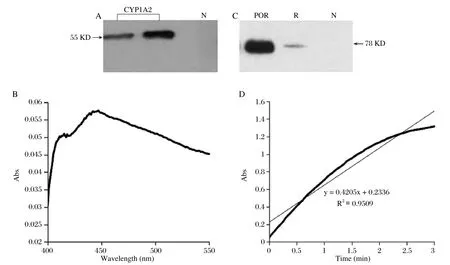
Fig. 2 Heterologous expression of recombinant CYP1A2 and POR cDNA in sf9 cells. Immunoblots of CYP1A2 (A)and POR (C). B: CO difference spectroscopy analysis of CYP1A2. D: cytochrome c assay of POR. N: negative. R: prepared rat liver microsomes.
To establish the optimal conditions of the heme precursors (hemin, 5-ALA and Fe3+)for expression,CYP1A2 and POR expression were measured in various combination of 0.1 mM 5-ALA, 0.1 mM Fe3+or 2 μg/ml hemin. Our results showed that compared with adding 5-ALA, Fe3+or hemin alone, co-adding 5-ALA and Fe3+could improve CYP1A2 expression significantly (35%, 51% and 35% higher, respectively)(Fig. 3A and 3B). However, no significant difference was found when adding 5-ALA, Fe3+or hemin alone.The POR expression level was about double control(adding nothing)when 5-ALA and/or Fe3+were added(Fig. 3C and 3D). Similarly, the POR specific activities in 5-ALA, Fe3+alone or together were 7.1, 4.5, 5.4 times higher than adding no factors (Table 1). Compared with the addition of 5-ALA or Fe3+alone, the addition of both together did not increase the expression level and activity of POR (P > 0.05), which differed from CYP1A2.

Fig. 3 Effects of different factors on CYP1A2 and POR expression. A: CYP1A2. B: POR Microsomal proteins (5 μg)of each sample were used. The results are expressed as western gray scale values (mean±SD; n = 3). *P < 0.05, compared with the corresponding groups (B), or compared with the group without adding factors (D).
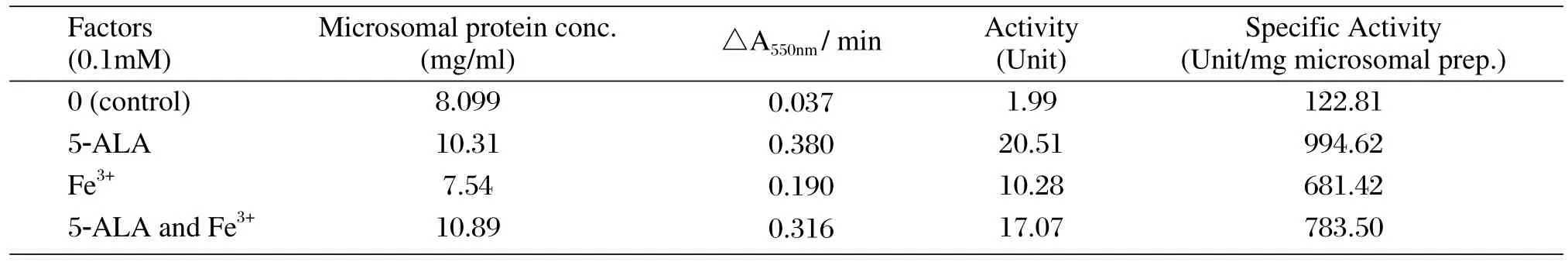
Table 1 Effects of different factors on POR activity
Effects of different virus ratios on coexpression of CYP1A2 and POR
CYP450s and POR could be co-expressed in sf9 cells by co-infecting with two recombinant viruses,bvCYP450s and bvPOR. This method had the advantage that it controlled the CYP/POR ratio by changing the ratio of bvCYP450s to bvPOR[10]. To obtain an optimal ratio of CYP/POR recombinant virus, we tried different ratios of bvCYP/bvPOR to investigate their effects on co-expression. Immunoblot analysis revealed that the bvCYP/bvPOR ratio affected the co-expression greatly. It showed that CYP1A2 and POR expression levels were significantly higher when the CYP1A2:POR virus ratio was 3:1 compared to other ratios (Fig. 4A and 4B).
Effects of different factors on co-expression of CYP1A2 and POR
To optimize the conditions for co-expression of CYP1A2 and POR, the CYP1A2 : POR virus ratio (3:1)was selected to detect the effects of various concentrations of 5-ALA (0.1-0.6 mM)and/or ferric citrate (0.1 mM)on the co-expression. Immunoblot analysis showed that low concentrations of 5-ALA (0.1 mM, 0.35 mM)favored the co-expression of CYP1A2 and POR. 5-ALA at both concentrations resulted in higher expression than did 0.6 mM 5-ALA. Unlike the effect of 5-ALA and Fe3+co-addition on the CYP1A2 or POR expression alone, this co-addition could not increase the CYP1A2 and POR co-expression level which was significantly lower than that induced by adding 5-ALA or Fe3+alone (P < 0.05). (Fig. 5A and 5B).
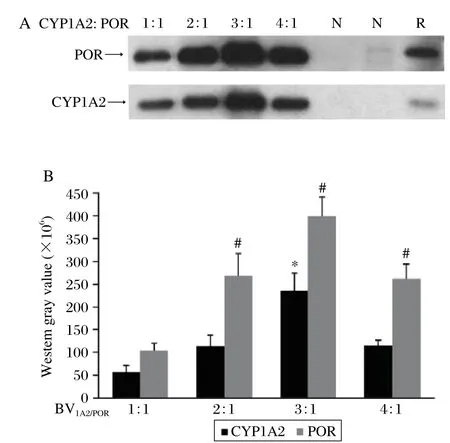
Fig. 4 Co-expression of CYP1A2 and POR using different virus ratios. Microsomal proteins (5 μg)of each sample were used. The western blot results (A)were expressed by western gray scale values (B)(mean±SD; n = 3), which defined the CYP1A2:POR virus ratio of 1:1 as the control, *P <0.05 of CYP1A2, #P < 0.05 of POR.
DISCUSSION
Because of the importance of CYP450s in metabolizing endogenous and exogenous compounds,attempts have been made to express them in heterologous systems, including mammalian cells, yeasts and bacteria[16,17]. However, current expression systems for producing eukaryotic P450s are hampered by serious drawbacks such as ① the low efficiency of production; ② the requirement of expensive materials and facilities, as well as sophisticated methods; ③ the extensive degradation of enzyme by the expression host or even the absence of biological activity. The baculovirus/sf9 system has been considered most efficient expression system. But, sf9 cells have a noted deficiency in CYP450s expression, namely their incorporation of heme. This problem can be partially resolved by adding heme precursors into the culture medium[18,19].
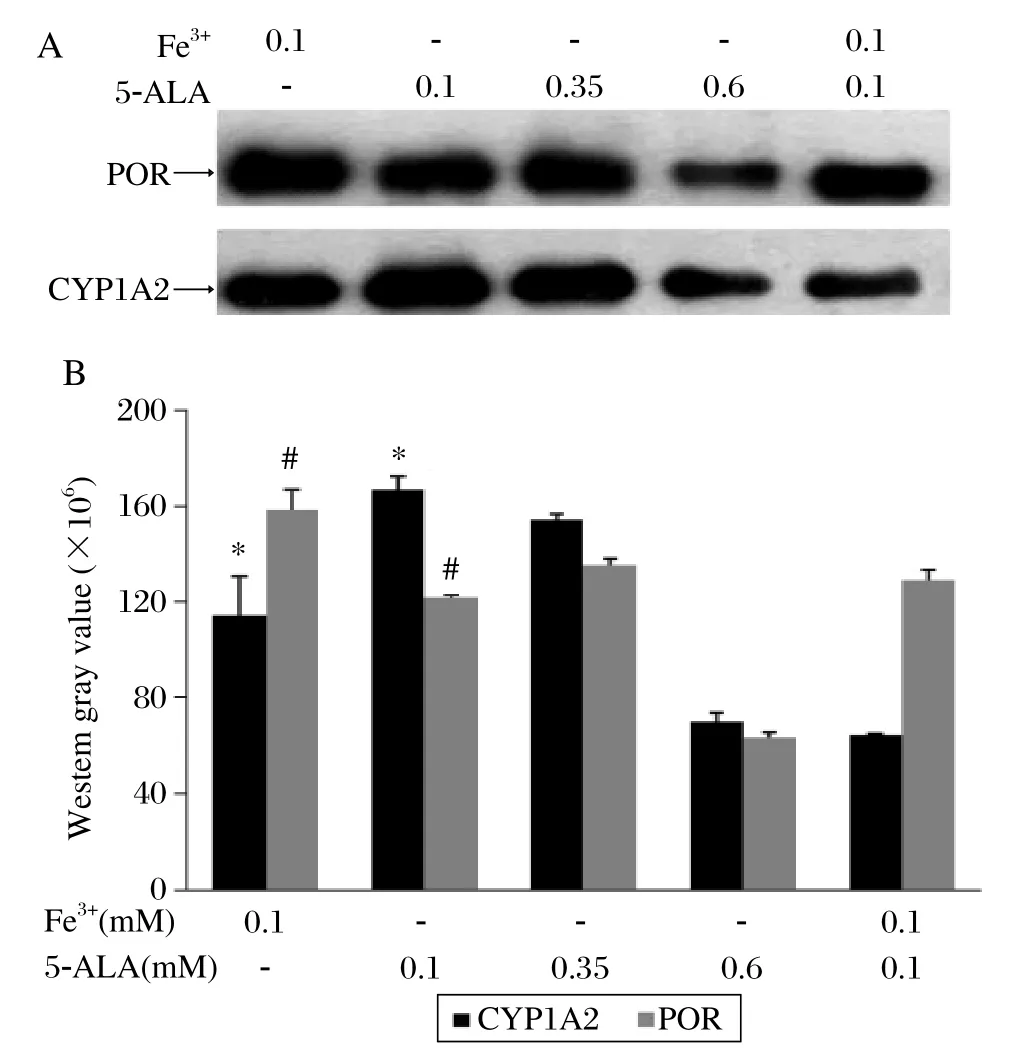
Fig. 5 Effects of different factors on co-expression of CYP1A2 and POR. Microsomal proteins (5 μg)of each sample were used. The western blot results (A)were expressed by western gray scale values (B)(mean±SD; n = 3). *P < 0.05 of CYP1A2, #P < 0.05 of POR, both compared with the group treated with 0.1 mM Fe3+ and 0.1 mM 5-ALA.
Hemin is the oxidized form (Fe3+)of heme. It can increase heme biosynthetic enzyme activity and stimulate other enzymes in the heme biosynthetic pathway. Hemin was used to express functional CYP1A1[18], CYP2A13[11], CYP2E1[12], CYP2S1[20]in baculovirus expression systems. However, hemin may be toxic to sf9 cells that have been in culture for several months[8]. 5-ALA is the first compound in the porphyrin synthesis pathway that leads to heme in mammals and to chlorophyll in plants, and the last step of this pathway is the combination of protoporphyrin IX with iron. It has received attention for its great potential as a holodiagnostic agent in clinical practice, and as a selective and biodegradable herbicide, insecticide and growth promoting factor[21].It also has medical applications in photodynamic cancer therapy and tumor diagnosis[22]. Additionally,5-ALA is the first committed intermediate of the heme biosynthesis pathway, which has been used in the medium of E.coli to express CYP450 1A1[23]and its allelic variants[24], CYP21, CYP1A2[7], as well as co-expression of CYP1A1 variants and POR[10]in sf9 cells.
In our study, CYP450s in sf9 cells were expressed successfully after adding hemin, 5-ALA and (or)Fe3+. The highest expression level of CYP1A2 in experiments using the combined addition of 5-ALA and Fe3+revealed that the sf9 cells had cell substructure integrity and all the enzyme pathways necessary for heme synthesis. Our results also revealed that as the addition of Fe3+improved the expression, it might be that iron is essential for the synthesis pathway. For this reason the cell medium was supplemented with the addition of Fe3+as an iron source. Also the level of CYP1A2 with the co-addition of 5-ALA and Fe3+was higher than that with hemin alone. The reason might be the toxicity of hemin to sf9 cells. Moreover,the method used to dissolve the hemin (dissolved in 0.2 M NaOH and 50% ethanol)might affect the enzyme expression because NaOH and ethanol could change the pH value of the culture medium, which subsequently decreased the availability of sf9 cells and then the protein expression. Although heme was not essential for POR expression and activity, these were significantly higher with 5-ALA or (and)Fe3+than without any factors. As we mentioned above, 5-ALA is a growth promoting factor and it may promote the growth of sf9 cells. Thus it can increase both POR and CYP1A2 expression levels and activities.
The electron transfer chain, usually NADPH and POR, plays an important role in reconstituting catalytically active CYP450 systems for metabolic activation. Therefore co-expression of CYP450 and POR in the baculovirus/sf9 system was studied extensively because this system could enable high production of the unmodified, enzymatically active native CYP450 in the microsomal membrane.Previous studies revealed successful expression of CYP1A1, CYP2A1 and CYP2A6, but their coexpression levels with POR were 50%-60% lower than their expression alone[10,25,26]. As discussed in these papers, it was assumed that POR expression, probably via heme degradation, directly influenced the CYP450 holoenzyme level. One study on co-expression of CYP2D6 and POR using an expression vector containing both CYP2D6 and POR cDNAs revealed that high levels of POR having an adverse effect on CYP450 expression might be a general property of coexpression in sf9 cells[27,28]. During the co-expression,degradation of the expressed holo-CYP1A1[10],CYP2E1 and CYP3A1[29]could be circumvented, at least partially, by addition of heme ligand or inhibitors such as imidazole and α-naphthoflavone, because these inhibitors can strongly bind to microsomal CYP by a direct ligation of an azole nitrogen with the iron atom of the CYP heme group[30]. The current study provided a new way to increase co-expression levels of CYP and POR by changing the CYP/POR ratio and combination of heme precursors. However, the co-expression level decreased with the simultaneous addition of 5-ALA and Fe3+, which did not affect the expression of CYP1A2 or POR alone. The mechanism of this phenomenon should be further explored.
[1]Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer 2006;6:947-60.
[2]Wang B, Zhou SF. Synthetic and natural compounds that interact with human cytochrome P450 1A2 and implications in drug development. Curr Med Chem 2009;16:4066-218.
[3]Gunes A, Dahl ML. Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics 2008;9:625-37.
[4]McKean C, Tang L, Tang M, Billam M, Wang Z,Theodorakis CW, et al. Comparative acute and combinative toxicity of aflatoxin B1 and fumonisin B1 in animals and human cells. Food Chem Toxicol 2006;44:868-76.
[5]Miller WL. Minireview: regulation of steroidogenesis by electron transfer. Endocrinology 2005;146:2544-50.
[6]Miller WL, Huang N, Agrawal V, Giacomini K M.Genetic variation in human P450 oxidoreductase. Mol Cell Endocrinol 2009;300:180-4.
[7]Harnastai IN, Gilep AA, Usanov SA. The development of an efficient system for heterologous expression of cytochrome P450s in Escherichia coli using hemA gene co-expression. Protein Expr Purif 2006;46:47-55.
[8]Gonzalez FJ, Kimura S, Tamura S, Gelboin HV.Expression of mammalian cytochrome P450 using baculovirus. Methods Enzymol 1991;206:93-9.
[9]Duarte MP, Palma BB, Gilep AA, Laires A, Oliveira JS, Usanov SA, et al. The stimulatory role of human cytochrome b5 in the bioactivation activities of human CYP1A2, 2A6 and 2E1: a new cell expression system to study cytochrome P450-mediated biotransformation (a corrigendum report on Duarte et al. (2005)Mutagenesis 20, 93-100). Mutagenesis 2007;22:75-81.
[10]Schwarz D, Kisselev P, Honeck H, Cascorbi I, Schunck WH, Roots I. Co-expression of human cytochrome P4501A1 (CYP1A1)variants and human NADPH-cytochrome P450 reductase in the baculovirus/insect cell system. Xenobiotica 2001;31:345-56.
[11]Zhou DS, Linnenbach AJ, Liu RF, Luzietti RA,Harris JJ, Booth-Genthe CL, et al. Expression and characterization of dog cytochrome P450 2A13 and 2A25 in baculovirus infected insect cells. Drug Metab Dispos 2010; Epub ahead of print.
[12]Chen W, Peter RM, McArdle S, Thummel KE, Sigle RO, Nelson SD. Baculovirus expression and purification of human and rat cytochrome P450 2E1. Arch Biochem Biophys 1996;335:123-30.
[13]Patten CJ, Smith TJ, Murphy SE, Wang MH, Lee J,Tynes RE, et al. Kinetic analysis of the activation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by heterologously expressed human P450 enzymes and the effect of P450-specific chemical inhibitors on this activation in human liver microsomes. Arch Biochem Biophys 1996;333:127-38.
[14]Wang SL, He XY, Shen J, Wang JS, Hong JY. The missense genetic polymorphisms of human CYP2A13:functional significance in carcinogen activation and identification of a null allelic variant. Toxicol Sci 2006;94:38-45.
[15]He XY, Shen J, Hu WY, Ding X, Lu AY, Hong JY.Identification of Val117 and Arg372 as critical amino acid residues for the activity difference between human CYP2A6 and CYP2A13 in coumarin 7-hydroxylation.Arch Biochem Biophys 2004;427:143-53.
[16]Guengerich FP, Gillam EM, Shimada T. New applications of bacterial systems to problems in toxicology. Crit Rev Toxicol 1996;26:551-83.
[17]Yun CH, Yim SK, Kim DH, Ahn T. Functional expression of human cytochrome P450 enzymes in Escherichia coli. Curr Drug Metab 2006;7:411-29.
[18]Buters JT, Shou M, Hardwick JP, Korzekwa KR,Gonzalez FJ. cDNA-directed expression of human cytochrome P450 CYP1A1 using baculovirus. Purification,dependency on NADPH-P450 oxidoreductase, and reconstitution of catalytic properties without purification.Drug Metab Dispos 1995;23:696-701.
[19]Gonzalez FJ, Korzekwa KR. Cytochromes P450 expression systems. Annu Rev Pharmacol Toxicol 1995;35:369-90.
[20]Wang SL, He XY, Hong JY. Human cytochrome p450 2s1: lack of activity in the metabolic activation of several cigarette smoke carcinogens and in the metabolism of nicotine. Drug Metab Dispos 2005;33:336-40.
[21]Fukuda H, Casas A, Batlle A. Aminolevulinic acid:from its unique biological function to its star role in photodynamic therapy. Int J Biochem Cell Biol 2005;37:272-6.
[22]Nishikawa S, Murooka Y. 5-Aminolevulinic acid:production by fermentation, and agricultural and biomedical applications. Biotechnol Genet Eng Rev 2001;18:149-70.
[23]Zhang ZY, Fasco MJ, Huang L, Guengerich FP,Kaminsky LS. Characterization of purified human recombinant cytochrome P4501A1-Ile462 and -Val462:assessment of a role for the rare allele in carcinogenesis.Cancer Res 1996;56:3926-33.
[24]Chernogolov A, Behlke J, Schunck WH, Roots I, Schwarz D. Human CYP1A1 allelic variants:baculovirus expression and purification, hydrodynamic,spectral, and catalytical properties and their potency in the formation of all-trans-retinoic acid. Protein Expr Purif 2003;28:259-69.
[25]Tamura S, Korzekwa KR, Kimura S, Gelboin HV,Gonzalez FJ. Baculovirus-mediated expression and functional characterization of human NADPH-P450 oxidoreductase. Arch Biochem Biophys 1992;293:219-23.
[26]Chen L, Buters JT, Hardwick JP, Tamura S, Penman BW, Gonzalez FJ, et al. Coexpression of cytochrome P4502A6 and human NADPH-P450 oxidoreductase in the baculovirus system. Drug Metab Dispos 1997;25:399-405.
[27]Paine MJ, Gilham D, Roberts GC, Wolf CR. Functional high level expression of cytochrome P450 CYP2D6 using baculoviral expression systems. Arch Biochem Biophys 1996;328:143-50.
[28]Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I. Clin Pharmacokinet 2009;48:689-723.
[29]Yang KH, Choi YH, Lee U, Lee JH, Lee MG.Effects of cytochrome P450 inducers and inhibitors on the pharmacokinetics of intravenous furosemide in rats: involvement of CYP2C11, 2E1, 3A1 and 3A2 in furosemide metabolism. J Pharm Pharmacol 2009;61:47-54.
[30]Sergent T, Dupont I, Jassogne C, Ribonnet L, van der Heiden E, Scippo ML, et al. CYP1A1 induction and CYP3A4 inhibition by the fungicide imazalil in the human intestinal Caco-2 cells-comparison with other conazole pesticides. Toxicol Lett 2009;184:159-68.
 THE JOURNAL OF BIOMEDICAL RESEARCH2010年3期
THE JOURNAL OF BIOMEDICAL RESEARCH2010年3期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- HGF percutaneous endocardial injection induces cardiomyocyte proliferation and rescues cardiac function in pigs☆
- CYP17 T27C polymorphism and prostate cancer risk:a meta-analysis based on 31 studies
- RNAi knockdown of C-erbB2 expression inhibits salivary gland adenoid cystic carcinoma SACC-83 cell growth in vitro
- Elevated expression of mature miR-21 and miR-155 in cancerous gastric tissues from Chinese patients with gastric cancer
- Identification of the metabolites of polybrominated diphenyl ether 99 and its related cytochrome P450s☆
- Weighted Markov chains for forecasting and analysis in Incidence of infectious diseases in jiangsu Province, China☆
