Investigation of Nitration Processes of iso-Octanol with Mixed Acid in a Microreactor*
SHEN Jiani (沈佳妮), ZHAO Yuchao (趙玉潮), CHEN Guangwen (陳光文),** and YUAN Quan (袁權(quán))
?
Investigation of Nitration Processes of-Octanol with Mixed Acid in a Microreactor*
SHEN Jiani (沈佳妮)1,2, ZHAO Yuchao (趙玉潮)1, CHEN Guangwen (陳光文)1,**and YUAN Quan (袁權(quán))1
1Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China2Graduate University, Chinese Academy of Sciences, Beijing 100049, China
In this paper, the nitration characteristic of alcohols with mixed acid for the synthesis of energetic materials in a stainless steel microreactor was investigated experimentally. The nitration of-octanol with HNO3-H2SO4mixed acid was chosen as a typical model reaction which involved fast and strong exothermic liquid-liquid heterogeneous reaction process. The influences of mixed acid composition, flow rate, organic/aqueous flow ratio and reaction temperature have been investigated. The results indicated that the reaction could be conducted safely and stably in the microreactor at 25-40°C, which are enhanced compared to 15°C or below for safe operating conditions in the conventional reactors. Moreover, the 98.2% conversion of-octanol could be obtained and no by-products were detected in all cases.
nitration, 2-ethylhexyl nitrate, microchannel, micromixer, microfluidic
1 Introduction
Many energetic material syntheses involve fast and highly exothermic liquid-liquid heterogeneous reaction process and have explosive potential in conventional reactors, such as nitration of alcohols. They are generally temperature sensitive and have to be considered as inhibited reactions. The nitrates can easily volatilize and undergo a self-sustaining decomposition with an intensive heat release. Once the dissipation of heat is out of control, the temperature runaway and even explosion of the energetic materials can be induced [1]. As the highly efficient mixing and excellent heat control are difficult to be performed in the conventional reactors [2], the nitration of alcohols with HNO3-H2SO4mixed acid is restricted within lower temperature and slow reaction regime due to its critical safety. Even so, the accidents still occur due to highly explosion of the reaction mixture. Moreover, slow heat and mass transfer rates can lead to the formation of undesirable by-products and the low productivity [3].
One way to increase the process safety and efficiency of fast highly exothermic reaction is to miniaturize the internal volume of reactor systems by employing the microreactor technology [4-8]. Because of the flow structures in sub-millimeter dimensions inside the microreactor, the mass and heat transfer can be greatly intensified compared to the conventional batch reactor, and this can offer the possibility to carry out reactions under isothermal conditions, such as nitration [9], sulphonation [10] and oxidation [11],. In addition, the smaller hold-up volume of microreactor increases the high operational safety to handle the syntheses of energetic materials and to investigate their chemical behaviours [6, 12]. The published literatures on nitration processes in the microreactor have demonstrated the safety characteristic of microreactor and the possibility of optimizing reactions by studying the effect of dependent parameters on reaction rates [9, 13-15]. Ramshaw. [12] and Dummann. [6] investigated the mechanism of enhanced mass transfer rate, and Halder. [15] testified that nitration of toluene can be controlled within intrinsic kinetics regime in the microreactor.
In this paper, synthesis of 2-ethylhexyl nitrate (2-EHN) was identified as a typical temperature sensitive model reaction to demonstrate the characteristic of the nitration process of alcohols with HNO3-H2SO4mixed acid for the synthesis of energetic materials in the stainless steel microreactors, which involves the fast and highly exothermic liquid-liquid heterogeneous reaction process. In fact, 2-EHN is also an important cetane number improver of diesel fuel which can reduce hydrocarbon emissions and NO formation, smooth engine operation and improve cold start [16]. The reaction is usually conducted with alkoxyalcohol as the additives in huge cooler systems to reduce the accumulation of heat and avoid the temperature runaway in conventional batch process. However, the additives bring more by-products, which lower the purity and increase the load in subsequent refining steps. For the mixed acid nitration, besides the main reaction [Eqs. (1) and (2)], the strong oxidizing ability of mixed acid may lead to the product degradation. Also, the high activity of sulfuric acid can cause the hydrolysis of product [Eq. (3)], resulting in the low conversion [3].



Considering the advantages of the microreactor, the direct nitration of-octanol with mixed acid was conducted at or above room temperature (25°C) in our study to investigate the potential of the microreactor from both process efficiency and safety point of view.
2 Experimental
The structure of the stainless steel microreactors used in this experiment is shown in Fig. 1. Etched plate had the same inlet configuration on both sides between point 1/point 2 and point 3 [see Fig. 1 (b)]. Parent channel was divided equally into two arc shaped sub-channels stage by stage until 16 parallel channels were generated. Organic compound and mixed acid were fed at point 1 and point 2, respectively. The two flows passed through each inlet zone independently and converged at orifices (point 3) on one side of microreactor plate. Reactants mixed and reacted along the microchannels between point 3 and point 4 with the dimension of every microchannel of 0.5 mm×0.5 mm×78 mm. In this reaction process, the total microreactor system was heated by electric- heating method. The mixture of reaction product effused the microreactor at point 4.

Figure 1 Structure of microreactor
Figure 2 shows a schematic diagram of the experimental setup. Two HPLC pumps were used to feed-octanol (AR, Tianjin Damao Chemical Reagent Factory) and mixed acid (both sulfuric acid and nitric acid are AR, Shenyang chemical reagent factory) through two inlets (points 1 and 2) into the microchannel reactor, respectively. The flow rate and composition of mixed acid were regulated to optimize the operating conditions. Short Teflon tubes with an inner diameter of 3 mm were used to connect all modules. Following the reaction section, there was a cold trap in which the reacting liquid-liquid mixtures were rapidly diluted and cooled to 0°C with ice-water mixture to terminate the reaction instantaneously. Moreover, organic- aqueous two phases were separated quickly. The organic phase was washed by water and Na2CO3(AR, Tianjin Kermel Chemical Reagent Co., Ltd.) solution up to neutralization. The composition of organic phase was identified and quantified by gas chromatograph (GC) with nitrogen as the carrier gas, and a flame ionization detector (FID), an OV-101 capillary column (0.25 μm film thickness, 0.25×50 m) were used. An internal standardization method was used following the procedure given by Grob [17]. Fig. 3 showed the graph of the gas chromatograph. The concentration of the unreacted-octanol was calculated from the concentration of 2-ethylhexyl nitrate since no other products or by-products were found to be formed in this process as shown in Fig. 3. The conversion of-octanol was calculated as the following equation.

Figure 2 Schematic diagram of experimental setup 1—organic phase tank; 2—aqueous phase tank; 3—HPLC pump; 4—check valve; 5—microreactor; 6—product collection tank; 7—thermocouple

whereis the conversion (%, by mass) of-octanol;M-octanoland2-EHNare the molecular weight of-octanol and 2-EHN, respectively;w-octanoland2-EHNare the mass fraction of-octanol and 2-EHN in the product organic phase, respectively.

Figure 3 Graph of the gas chromatograph
Liquid hourly space velocity (LHSV) can be defined in terms of Eq. (5):

Residence time can be calculated based on LHSV as following Eq. (6):
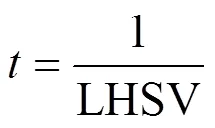
whereaqandorare the aqueous flow velocity and the organic flow velocity, respectively, m3·s-1;Ris the microreactor volume, m3;denotes the residence time of organic and aqueous two phases in microreactor, s.
Zaldivar. [18, 19] has indicated that the nitration of aromatics falls into the fast reaction regime at H2SO4strengths of 70%-80% (by mass) in mixed acid. However, the H2SO4strengths of mixed acid are larger than 80% (by mass) in our experiments. In addition, alcohols are more easily nitrated by mixed acid under the same conditions compared to the aromatics. Thus, in the-octanol nitration liquid-liquid heterogeneous process with HNO3-H2SO4mixed acid, the reaction rate can be calculated by an interfacial nitration model aimed at fast reaction regimes, and the average reaction rate is adopted in this paper. This model assumes that organic nitration occurs in the aqueous phase close to the interface and is controlled by mass transfer of organic phase across the interface of liquid-liquid two phases, and the reaction occurs within the mixed acid phase. The average reaction rate based on the volume of the microreactor can be written as:




whereρ-octanolis the density of-octanol, kg·m-3.
Combining Eqs. (7), (8) and (9), Eq. (10) can be deduced:

3 Results and discussion
In the nitration of-octanol with mixed acid, chemical reaction and mass transfer are simultaneously carried out and both affected by the mixed acid composition. In order to investigate the nitration process of-octanol in the microreactor, a series of experiments were performed with different operating conditions.
3.1 Effect of sulfuric acid concentration in the mixed acid
To study the influence of sulfuric acid concentration, the nitration process of-octanol was investigated at room temperature with 2% and 15% H2O (by mass) in the mixed acid, respectively. In this part, the mole ratio of nitric acid to-octanol and LHSV were fixed to 1.0 and 1000 h-1, respectively. The experimental results in Fig. 4 show that the conversion of-octanol gradually increased with sulfuric acid concentration in the mixed acid for 15% H2O (by mass) within the same residence time. However, this effect was reduced when the content of water decreases to 2% (by mass) especially for the sulfuric acid concentration in the range of 67% to 86% (by mass).



3.2 Effect of water content in the mixed acid




Table 1 Sulfuric acid strength of mixed acid before and after reaction
3.3 Effect of LHSV
In order to investigate the effect of LHSV on the nitration process of-octanol, the experiments were carried out using two kinds of typical mixed acid composition at room temperature. Their mass composition was 58% H2SO4and 26% HNO3, and 74% H2SO4and 24% HNO3, respectively. The average reaction rate is plotted against LHSV in Figs. 6 and 7. It could be seen that the average reaction rate was directly proportional to LHSV for both reaction systems, implicating these nitration processes were still limited by mass transfer in the microreactor. In fact, the effect of LHSV was more remarkable in the mixed acid with higher sulfuric acid strength because the reaction rate was faster and the nitration process was more greatly limited by mass transfer performance of liquid-liquid immiscible two phases. According to our previous research [24], the flow regimes selected for the present investigation are mainly the parallel flow with smooth interface, the parallel flow with wavy interface, and even the chaotic thin striations flow in this microreactor. The hydrodynamic characteristics of mass transfer in these flow regimes have been systematically investigated. The overall volumetric mass transfer coefficients obtained are more than two or three orders of magnitude higher than those in traditional liquid-liquid large scaled contactors [25]. The interfacial area between two immiscible fluids stretches, deforms, and folds with the increase of LHSV, as well as the surface renewal velocity is enhanced, making the interphase mass transfer more effective, which significantly intensifies mass transfer process and increases the overall volumetric mean mass transfer coefficient. Therefore, the average reaction rate increased with the velocity in the mass transfer controlled systems. Obviously, this effect on the mass transfer was relatively severe in the mixed acid system with higher sulfuric acid strength. Moreover, when LHSV was high enough, the reaction would be controlled by kinetics rather than mass transfer, so average reaction rate achieved limit value as shown in Fig. 6. In fact, the higher-octanol conversion could be obtained with lower LHSV in these reaction systems due to the longer residence time in the microreactor.




3.4 Effect of reaction temperature
To ensure the safety of the nitration process of-octanol, the reaction temperature is usually maintained below 15°C in common commercial process [26]. In this part, the experiment was carried out in typically fast reaction regime with the mass composition of 74% H2SO4and 24% HNO3, and the microreactor temperatures were varied from 25°C to 40°C as shown in Fig. 8. It was observed that the conversion of-octanol increased with the reaction temperature due to the increase of the generation rate of nitronium ion. Moreover, the overall nitration reaction process performed safely and stably, and no other by-products were found, whereas oxidation occurred even at lower temperature in the conventional reactor. The faster heat transfer rate coupled with smaller internal reaction volumes of the microreactor increased the ability of temperature control, which not only ensured the safety at higher temperatures, but also prevented temperature-induced side reactions and thermal decomposition of 2-ethylhexyl nitrate. Ultimately, the selectivity and the purity were increased compared to the corresponding traditional-octanol nitration reaction process.
3.5 Effect of HNO3/C8H17OH mole ratio

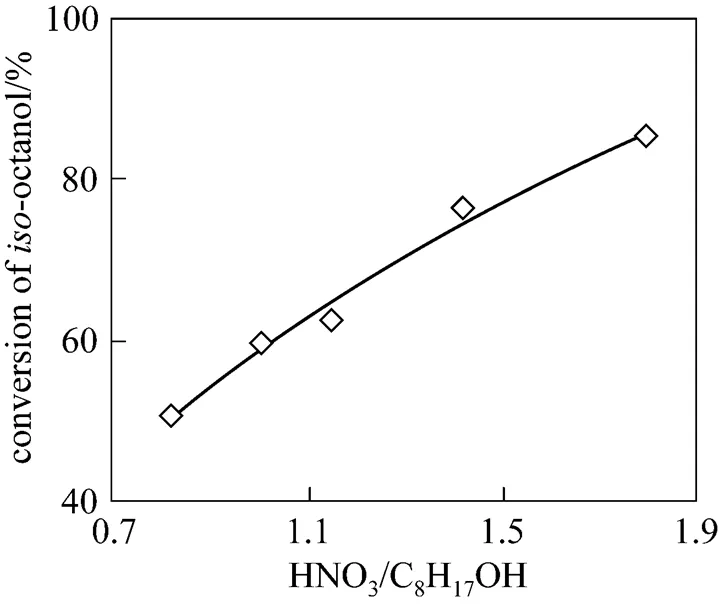

3.6 The optimization of reaction conditions
On the basis of studying the effect of independent parameters on synthesis of 2-EHN in the microreactor, a series of experiments were carried out to optimize the reaction conditions to offer more information for industrialisation. The products from the microreactor with or without cold trap section were respectively collected to compare quantitatively the conversion of the-octanol under same reaction conditions, as shown in Table 2. It was shown that the conversion of the-octanol in the microreactor without cold trap section was higher than with cold trap section due to the longer reaction time, indicating the tube connected to the output or the long channels of the microreactor were in favor of enhancing the conversion of the-octanol. In fact, it is observed that the reaction is still mainly accomplished in the microreactor system, ensuring the safety of reaction process and the purity of product. It can be seen that the conversion reached 98.2% in the microreactor without cold crap when the reaction temperature was 35°C, the residence time was 7.2 s, and the HNO3/C8H17OH mole ratio was 1.5. So the nitration of-octanol can be carried out in the microreactor efficiently and safely.



Table 2 The conversion of iso-octanol under different reaction conditions
4 ConclusionS
The main objective of this paper is to demonstrate the feasibility and safety of the 2-ethylhexyl nitrate synthesis in the microreactor system, which is proposed to carried out fast exothermic liquid-liquid reaction for energetic material synthesis. The nitration of-octanol with mixed acid exhibits the typical features of the fast highly exothermic reaction, even potentially explosion or side reactions associated.
The characteristics of the nitration process were investigated by studying the effect of mixed acid composition and operating conditions on the conversion of-octanol and the average reaction rate. The results showed that the reaction could be almost completed in the microreactor system under some particular reaction conditions. Also, the reaction could be conducted safely and stably in the microreactor at 25-40°C, which are enhanced compared to 15°C or below for safe operating conditions in the conventional reactors. Moreover, no by-product was formed and 98.2% of the conversion of-octanol could be obtained. Microreactors with enhanced heat and mass transfer combined with small internal channel dimensions offer an excellent tool to reduce overall effort to commercial processes for fast exothermic liquid-liquid heterogeneous phase reaction. Based on the advantageous mentioned above, it can be accepted that the microreactor is one of the most suitable research and industrial instruments for fast exothermic liquid-liquid reaction.
Nomenclature
LHSV liquid hourly space velocity, h-1
molecular weight, g·mol-1
residence time, s
volume, m3
flow velocity, m3·s-1
mass fraction in the product organic phase, %
mass conversion of-octanol, %
density, g·m-3
Subscripts
aq aqueous
or organic
R reactor
1 ATC, “2-Ethylhexyl nitrate best practices manual”, http://www.atc-europe.org/publications.asp Document 79 (2004).
2 van Woezik, B.A.A., Westerterp, K.R., “Runaway behavior and thermally safe operation of multiple liquid-liquid reactions in the semi-batch reactor—The nitric acid oxidation of 2-octanol”,.., 41, 59-77 (2002).
3 Suppes, G.J., Dasari, M.A., “Synthesis and evaluation of alkyl nitrates from triglycerides as cetane improvers”,...., 42, 5042-5053 (2003).
4 Haswell, S.J., Middleton, R.J., O’Sullivan, B., Skelton, V., Watts, P., Styring, P., “The application of micro reactors to synthetic chemistry”,.., 5, 391-398 (2001).
5 Dummann, G., Quittmann, U., Gr?schel, L., Agar, D.W., W?rz, O., Morgenschweis, K., “The capillary-microreactor: A new reactor concept for the intensification of heat and mass transfer in liquid-liquid reactions”,., 79/80, 433-439 (2003).
6 J?hnisch, K., Hessel, V., L?we, H., Baerns, M., “Chemistry in microstructured reactors”,...., 43, 406-446 (2004).
7 Panke, G., Schwalbe, T., Stirner, W., Taghavi-Moghadam, S., Wille, G., “A practical approach of continuous processing to high energetic nitration reactions in microreactors”,-, 18, 2827-2830 (2003).
8 Chen, G.W., Yuan, Q., Li, H.Q., Li, S.L., “CO selective oxidation in a microchannel reactor for PEM fuel cell”,..., 101, 101-106 (2004).
9 Burns, J.R., Ramshaw, C., “A microreactor for the nitration of benzene and toluene”,..., 189, 1611-1628 (2002).
10 Müller, A., Cominos, V., Hessel, V., Horn, B., Schürer, J., Ziogas, A., J?hnisch, K., Hillmann, V., Gro?er, V., Jam, K.A., Bazzanella, A., Rinke, G., Kraut, M., “Fluidic bus system for chemical process engineering in the laboratory and for small-scale production”,..., 107, 205-214 (2005).
11 van Woezik, B.A.A., Westerterp, K.R., “The nitric acid oxidation of 2-octanol. A model reaction for multiple heterogeneous liquid-liquid reactions”,..., 39, 521-537 (2000).
12 Brivio, M., Verboom, W., Reinhoudt, D.N., “Miniaturized continuous flow reaction vessels: Influence on chemical reactions”,, 6, 329-344 ( 2006).
13 Ducry, L., Roberge, D.M., “Controlled autocatalytic nitration of phenol in a microreactor”,...., 44, 7972-7975 (2005).
14 Henke, L., Winterbauer, H., “A modular microreactor for mixed acid nitration”,..., 28, 749-752 (2005).
15 Halder, R., Lawal, A., Damavarapu, R., “Nitration of toluene in a microreactor”,., 125, 74-80 (2007).
16 Suppes, G.J., Rui, Y., Rome, A.C., Chen, Z., “Cetane-improver analysis and impact of activation energy on the relative performance of 2-ethylhexyl nitrate and tetraethylene glycol dinitrate”,...., 36, 4367-4404 (1997).
17 Grob, R.L., Modern Practice in Gas Chromatography, JohnWiley and Sons, New York, 181-184 (1977).
18 Zaldivar, J.M., Molgab, E., Ahos, M.A., Hernhndez, H., Westerterpc, K.R., “Aromatic nitrations by mixed acid. Slow liquid-liquid reaction regime”,..., 34, 543-559 (1995).
19 Zaldivar, J.M., Molgab, E., Ahos, M.A., Hernhndez, H., Westerterpc, K.R., “Aromatic nitrations by mixed acid. Fast liquid-liquid reaction regime”,..., 35, 91-105 (1996).
20 Schofield, K., Aromatic Nitration, Cambridge University Press (1980).
21 Cox, P.R., Strachan, A.N., “Two-phase nitration of toluene (part II)”,..., 4, 253-261 (1972).
22 Albright, L.F., Hanson, C., “Industrial and laboratory nitrations”,.., 178-181 (1975).
23 Quadros, P.A., Oliveira, N.M.C., Baptista, C.M.S.G., “Continuous adiabatic industrial benzene nitration with mixed acid at a pilot plant scale”,..., 108, 1-11 (2005).
24 Zhao, Y., Chen, G., Yuan, Q., “Liquid-liquid two-phase flow patterns in a rectangular microchannel”,., 52, 4052-4060 (2006).
25 Zhao, Y., Chen, G., Yuan, Q., “Liquid-liquid two-phase mass transfer in the T-junction microchannels”,., 53, 3042-3053 (2007).
26 Knapp, G.G., Baylerian, M.S., Seemuth, P.D., “Nitration process”, US Pat., 4479905 (1983).
2009-01-23,
2009-04-13.
the National Natural Science Foundation of China (20490208), the National High Technology Research and Development Program of China (2007AA030206), and the Open Fund of State Key Laboratory of Explosion Science and Technology, BIT (KFJJ06-1).
** To whom correspondence should be addressed. E-mail: gwchen@dicp.ac.cn
 Chinese Journal of Chemical Engineering2009年3期
Chinese Journal of Chemical Engineering2009年3期
- Chinese Journal of Chemical Engineering的其它文章
- Position Group Contribution Method for Estimation of Melting Point of Organic Compounds
- Process Intensification of VOC Removal from High Viscous Media by Rotating Packed Bed*
- Adsorption of Dye from Wastewater by Zeolites Synthesized from Fly Ash: Kinetic and Equilibrium Studies*
- Modeling of Isomerization of C8 Aromatics by Online Least Squares Support Vector Machine*
- Resolution of Ibuprofen Ester by Catalytic Antibodies in Water-miscible Organic-solvents*
- Reaction Characteristics of Asymmetric Synthesis of (2S,5S)-2,5-Hexanediol Catalyzed with Baker’s Yeast Number 6*
