Combination of Supercritical Fluid Extraction with Ultrasonic Extraction for Obtaining Sex Hormones and IGF-1 from Antler Velvet*
ZHOU Ran (周冉), LI Shufen (李淑芬) and ZHANG Dacheng (張大成)
?
Combination of Supercritical Fluid Extraction with Ultrasonic Extraction for Obtaining Sex Hormones and IGF-1 from Antler Velvet*
ZHOU Ran (周冉), LI Shufen (李淑芬)**and ZHANG Dacheng (張大成)
Key Laboratory for Green Chemical Technologies of the Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China
Supercritical CO2(SC-CO2) extraction technology and ultrasonic technology were used to extract two active sex hormones, estradiol and progesterone, and insulin-like growth factor-1 (IGF-1) from antler velvet. The effects of SC-CO2extraction condition on the extraction yield and content of sex hormones, the ultrasonic extraction condition on the content of IGF-1 and the SC-CO2extraction condition on the activity remaining of IGF-1 were studied. The optimal conditions were obtained. The experimental results showed that, in presence of 75% ethanol as the co-solvent, the mean yield and content of estradiol and progesterone were 87.67 pg·g-1and 1224.10 pg·g-1, 12.38 ng·g-1and 354.06 ng·g-1, respectively, with extraction pressure of 30 MPa, temperature of 35°C, extraction time of 30 min and CO2consumption of 15 L·g-1at the flow rate of 2.0 L·min-1. The highest content of IGF-1 was 7425.75 ng·g-1antler velvet residue, when the pH10 ammonia-ammonium chloride buffer solution was used as the solvent, the ratio of solvent to sample was 20/1 (volume/mass), the extraction temperature was 0-35°C, and the extraction time was 4×15 min. Under these conditions, 93.68% activity remaining of IGF-1 in the residue was obtained, while little IGF-1 activity exists in traditional residue. The experimental results indicate that the technology of SC-CO2with co-solvent is of advantage for getting high content sexual hormones and keeping high activity of IGF-1 in the residue, which can not be achieved by traditional extraction methods.
antler velvet, supercritical extraction, estradiol, progesterone, insulin-like growth factor-1
1 Introduction
Antler velvets are bony cranial appendages that develop on top of permanent frontal protuberances (the pedicles) in male deer, and undergo periodic replacement [1]. Traditional medical reports and clinical observations convincingly show that antler velvet is made of many components such as sphingomylein, ganglioside, estrone, estradiol, prostaglandins [2], collagen, amino acid-sugar combinations [3-4] and growth factors including insulin-like growth factor-1 [5], and epidermal growth factor [6], which may be the reasons of anti-aging.
Among these active components, the estrogen plays an important role to women in their whole life, the decreasing circulating levels of which is associated with menopause phase in a woman’s life [7]. Estradiol is the most active estrogen, which is the main drug in hormone replacement therapy (HRT). Progesterone is natural progestin, which can constantly perform hormone replacement therapy for postmenopausal women. It can effectively decrease the morbidity of the cardiovascular diseases when used in HRT. Moreover, it regulates the gene transcription about bone metabolism, and plays an important role in the treatment of postmenopausal osteoporosis. Insulin-like growth factor-1 (IGF-1) is a 7.5-kDa hormone that is regulated by growth hormone and is functionally similar to proinsulin [8-10]. Whetheror, IGF-1 can exhibit a certain biological function [11, 12], which has been shown to stimulate a pleiotrophic growth response affecting rates of protein synthesis, protein degradation, DNA synthesis and the transport of substrates.
According to literatures, the extraction of sex hormones from antler velvet is accomplished by vibrating, heating, or refluxing. For example, estradiol was extracted by hot refluxing method with methanol solvent [13]. Vibration extraction with acetone and methanol mixture solution was adopted to obtain sex hormone components [14], and then solid-liquid extraction with chloroform was performed for further HPLC analysis. Moreover, 90% methanol solution or pure dichloromethane and KOH (0.1 mol·L-1) were used in vibration extraction [2, 15] to get progesterone and estradiol, respectively, from antler velvet. These procedures have distinct drawbacks of high extraction temperature, time-consuming, handling of large volume of hazardous solvents and extended concentration steps. Therefore, it results in the loss of much blood and the degradation of active protein components. The extraction methods of IGF-1 were also limited in solvent extraction with acid solution because of its specific protein character [16]. This process has the advantage on getting many water-soluble components, but little sex hormone is obtained. It is an important research task to get more effective constituents from Chinese medical materials so that they could be utilized more efficiently and economically. In this respect, the approach for simultaneously obtaining sex hormones and IGF-1 from fresh antler velvet in short processing time will be an ideal technology.
One of the advantages of supercritical fluid extraction (SFE, compared with liquid extraction) is that it is a relatively rapid process because of the low viscosities and high diffusivities associated with supercritical fluids. The extraction can be selective to some extent by controlling the density of the medium and the extracted material is easily recovered by simply depressurizing, allowing the supercritical fluid to return to gas phase and evaporate, leaving no or little solvent residues. Carbon dioxide is the most commonly used supercritical solvent.
Ultrasound extraction, as a kind of new technique, produces cell disruption, particle size reduction and ultrasonic jet towards solid surfaces, leading to a greater contact area between solid and liquid phase, better access of solvent to valuable components, compared with traditional methods [17, 18]. This type of extraction has been applied to biological matrices such as plant materials [19-21] and even animal tissues [22], and has produced satisfactory results [23].
So far, it has not been reported in the literature that obtaining sex hormones and IGF-1 from antler velvet by the combination of supercritical fluid extraction with ultrasonic extraction. Hence, it is of interest to investigate the effects of SFE and ultrasound on the extraction of sex hormones and IGF-1 components. In this paper, the study on the conditions of extracting the active estradiol and progesterone constituents from antler velvet by SC-CO2and co-solvent and ultrasonic extraction for IGF-1 in the residue is carried out. The activity remaining of IGF-1 in the residue is analyzed as one of control indexes. The final aim is to get the active estrogen product and to keep the biological activity in antler velvet residue, so that the antler velvet materials can be better utilized.
2 Experimental
2.1 Materials
The raw material of fresh antler velvet (Cervus Nippon Temminck var. mantchurieus Sainhoe) was provided and identified by Xiuzheng Pharmaceutical Group (Jilin, China). It was sectioned at a thickness of 2-3 mm, and then was powdered into the size of 80-98 μm (160-180) mesh after freeze-drying (Christ Alpha1-2, Marin Christ, Germany).
Carbon dioxide stored in a cylinder with a purity of 99.9% was purchased from Liu Fang Gas Co. (Tianjin, China). The standard samples of estradiol (E2) and progesterone (Pro), all with purity greater than 99%, were obtained from Sigma Chemical Co. (USA). The ethanol and methanol were analytical grade (99%). The ethanol solvent used was filtered through a 0.45mm nylon membrane filter prior to utilization.
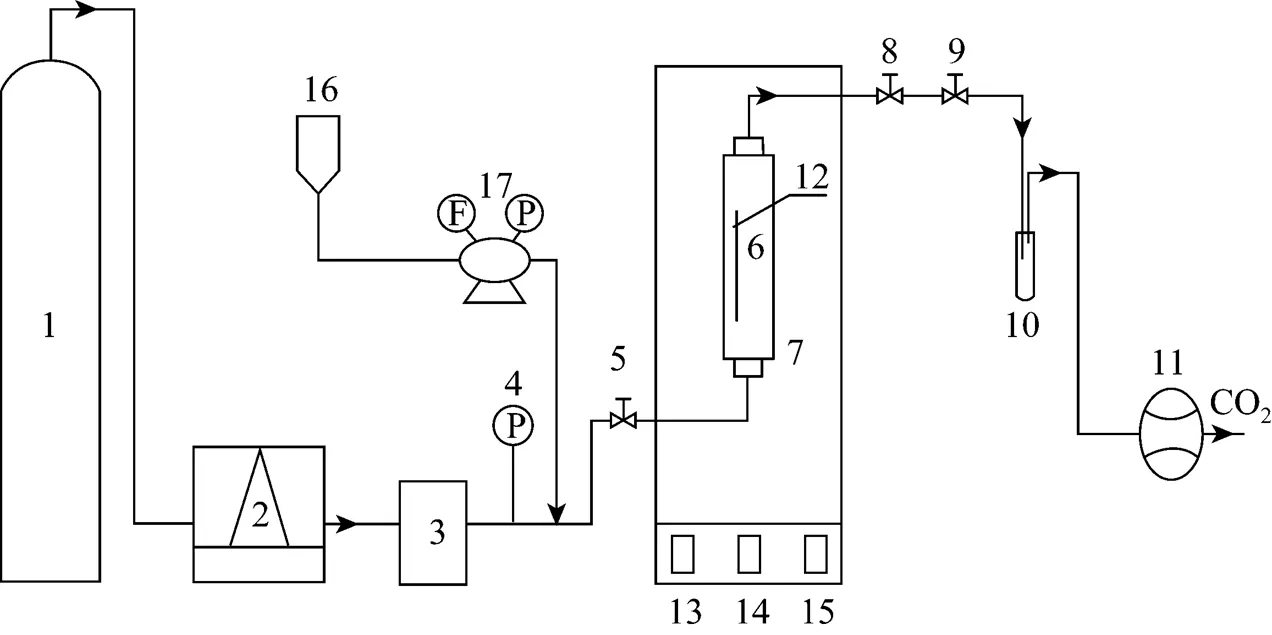
Figure 1 Schematic diagram of lab-scale SFE apparatus 1—CO2cylinder; 2—liquid-cooled bath; 3—gas booster pump; 4—pressure gauge; 5—in-let valve; 6—extraction column; 7—constant temperature oven; 8—out-let valve; 9—micrometer valve; 10—collection vial; 11—wet-test meter; 12—thermocouple; 13—oven temperature indicator; 14—column temperature indicator; 15—micrometer valve temperature indicator; 16—co-solvent tank; 17—co-solvent pump
2.2 Apparatus and procedure
2.2.1SC-CO and co-solvent extraction of estradiol and progesterone
The extraction was performed using a Speed SFE instrument (Applied Separations Inc., Allenton, PA, USA) equipped with a HPLC pump, shown schematically in Fig. 1. Four grams of antler velvet powder or its mixture with co-solvent was weighed and packed into the extraction column (capacity of 32 cm3). Liquid CO2was pressurized with a high-pressure pump and then charged into the extraction column to the desired pressure. The pressure was controlled to an accuracy of about 2% over the measuring range. The extraction column was heated with an oven and its temperature was indicated and controlled by the thermocouple to within ±1°C. After the column hold equilibrium at the desired constant pressure and temperature, the dynamic extraction process started, that is, the liquid co-solvent from the tank was pressurized with the co-solvent pump and charged into the extraction column together with carbon dioxide in a certain ratio. The SC-CO2and co-solvent with dissolved compounds passed through a heated micrometer valve, and were subsequently expanded to ambient pressure at room temperature. The flow rate of CO2was controlled at 2.0 L·min-1(ambient temperature and pressure) in this study. The extracts were collected in a trapping vial. Finally, both extracts and antler velvet residue were dried under vacuum equipment (ZK-30 (BS), Huabei Apparatus Co., Ltd., Tianjin, China) for further analysis and extraction.
2.2.2
A single factor experiment was considered to study the effect of kind of solvent, the ratio of solvent to sample (volume/mass), extraction temperature and time on IGF-1 content of the residue. An open rectangular ultrasonic cleaner bath (KQ-200KDE 40 kHz, 200 W) with useful volume of 6.0 L (internal dimensions: 30 cm′15 cm′15 cm) was used to carry out the extractions. The temperature was controlled and maintained to within ±3°C by circulating external water. The antler velvet residue of 1.0 g was mixed with the appropriate extraction solvent in a 40 ml glass vial that was immersed in water in the ultrasonic cleaning bath. The mixture was extracted for specific time (15, 30, 45, 60, 75, 105 and 120 min) for different ratios of solvent to sample (5︰1, 7︰1, 10︰1, 12︰1, 14︰1, 16︰1, 18︰1, and 20︰1) at different temperatures (0, 20, 35, 50, 65, and 80°C), and centrifuged at 9000 r·min-1for 10 min. The processes were carried out by sequentially varying the experimental parameters, one at a time, while all the other parameters remained fixed. The supernatants were collected for analysis.
2.3 Sample preparation and analysis methods
2.3.1
50 mg of supercritical fluid extract by supercritical carbon dioxide and co-solvent was dissolved with 10 ml methanol. The contents were vortexed vigorously for 1 min and then centrifuged at 5000 r·min-1for 10 min (TGL-16M, Xiangyi Centrifuge Instrument Co., Ltd., Hunan, China). The supernatant was collected and calibrated to 10 ml by methanol. 1 ml of the supernatant solution was dried in vacuum equipment [ZK-30 (BS), Huabei Apparatus Co., Ltd., Tianjin, China] and the final solid was redissolved in the sodium phosphate buffer (PBS) with pH 6.8 containing 0.2% bovine serum albumin (BSA). The sample solution was then stored at 4°C for radioimmunoassay (RIA).
The concentrations of estradiol (E2) and progesterone (Pro) were determined in conditioned media by radioimmunoassay using a commercially available estradiol kit (Union Medical and Pharmaceutical Technology Tianjin Ltd., China) and a progesterone kit (Chemclin Biotech Co., Ltd., Beijing, China), respectively. The incubation mixture consisted of 0.2 ml of125I-E2or125I-Pro, corresponding to 0.2 ml of E2(or 0.1 ml of Pro) standard solution containing 0, 5, 25, 100, 300, 750, or 2000 pg·ml-1(or 0, 0.1, 0.5, 2.0, 10, 30, or 150 ng·ml-1), and 0.2 ml of diluted antiserum. Each calibration point and unknown sample was assayed in duplicate; tubes corresponding to total activity (), binding capacity [(/)0] and nonspecific counts (/) were also prepared. After incubation for 1.5 h at 37°C, 1.0 ml of precipitating reagent was added, keeping the bulk suspension on a magnetic stirrer, and both suspension and RIA tubes refrigerated in an ice bath. After 10 min, the tubes were centrifuged at 24°C at 3000 r·min-1for 20 min, and each deposition was transferred to a vial for γ-counting.
2.3.2
The extract solution was centrifuged for 30 min (9000 r·min-1) at 4°C. The supernatant was collected and total volume was determined. The sample solution was stored at 4°C. The concentrations of IGF-1 were determined in conditioned media by radioimmunoassay using a commercially available IGF-1 kit (Tianjin Nine Tripods Medical and Bioengineering CO., Ltd., China).
3 Results and Discussion
3.1 SC-CO2 and co-solvent extraction of estradiol and progesterone
The structures of estradiol and progesterone are shown in Fig. 2. In our previously study, pure SC-CO2was first used to investigate the possibility of extracting estradiol and progesterone from antler velvet. However, non-polar CO2can not easily extract polar estradiol and progesterone from antler velvet.
Figure 2 The molecular structures of estradiol and progesterone
SC-CO2extraction in the presence of static co-solvent was further investigated, where ethanol solution was selected as a co-solvent, as it is nontoxic, cheap and easy to change its polarity with adding water. However, the experimental results (shown in Table 1) were still not satisfied although the extraction yields and the contents of estradiol and progesterone in the extracts greatly increased compared to those by single supercritical CO2extraction. Therefore, SC-CO2extraction in the presence of dynamic co-solvent was employed in order to enhance the extraction efficiency.
The experiments were carried out by sequentially varying the experimental parameters, one at a time, while all the other parameters remained fixed. The maximum extraction yield and content of estradiol and progesterone were considered as selected indexes. In all the experiments, the flow rate of CO2was set at 2.0 L·min-1.
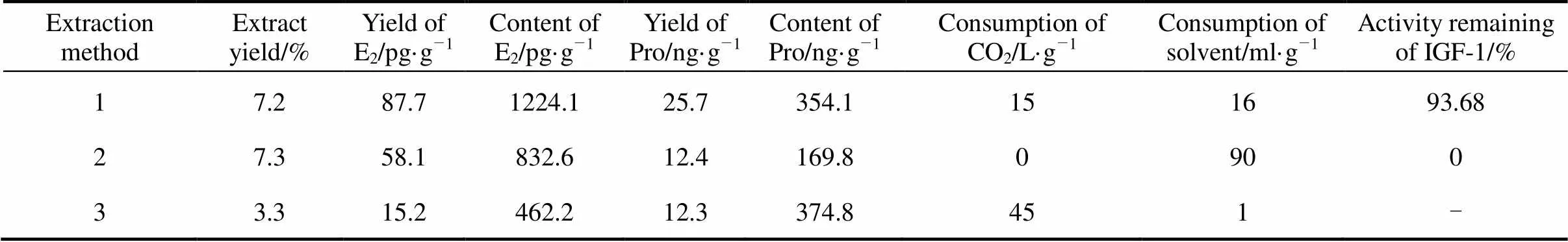
Table 1 Results of E2 and Pro obtained by different methods
Note:Method 1, this study (SC-CO2extraction with 75% ethanol as dynamic co-solvent at 35°C); Method 2, refluxing extraction with methanol as solvent for three times; Method 3, SC-CO2extraction with 85% ethanol as static co-solvent (previous study).
3.1.1
The experiments were carried out at constant extraction temperature of 35°C and extraction pressure of 30 MPa. It can be seen from Figs. 3 and 4 that the yield and content of estradiol are the highest when the concentration of 75% ethanol solution is used as the co-solvent and the yield and content of progesterone are the highest when 85% ethanol solution is used as the co-solvent. It is considered that the polarity of progesterone is less than that of estradiol, as estradiol has two hydroxyl groups and progesterone has one carbonyl. With increasing concentration of ethanol-water solution, the polarity would decrease, so that estradiol has better solubility in 75% ethanol-water solution than in 85%.
3.1.2
The effect of the flow rate of co-solvent was studied at a constant pressure of 30 MPa and temperature of 35°C while 75% ethanol solution was used as the co-solvent. The experimental results (Figs. 3 and 4) indicate that the yield and content of estradiol are the highest when the flow rate of co-solvent is 2.0 ml·min-1, and the yield and content of progesterone are the highest at the flow rate of co-solvent 1.5 ml·min-1. Therefore, the flow rate of co-solvent from 1.5 ml·min-1to 2.0 ml·min-1was collected in this study.
3.1.3
The experiments were carried out in the pressure ranging from 10 to 40 MPa at 35°C and 75% ethanol solution was used as the co-solvent. It can be seen from Figs. 3 and 4 that the yield and content of estradiol and progesterone are the highest at 30 MPa.
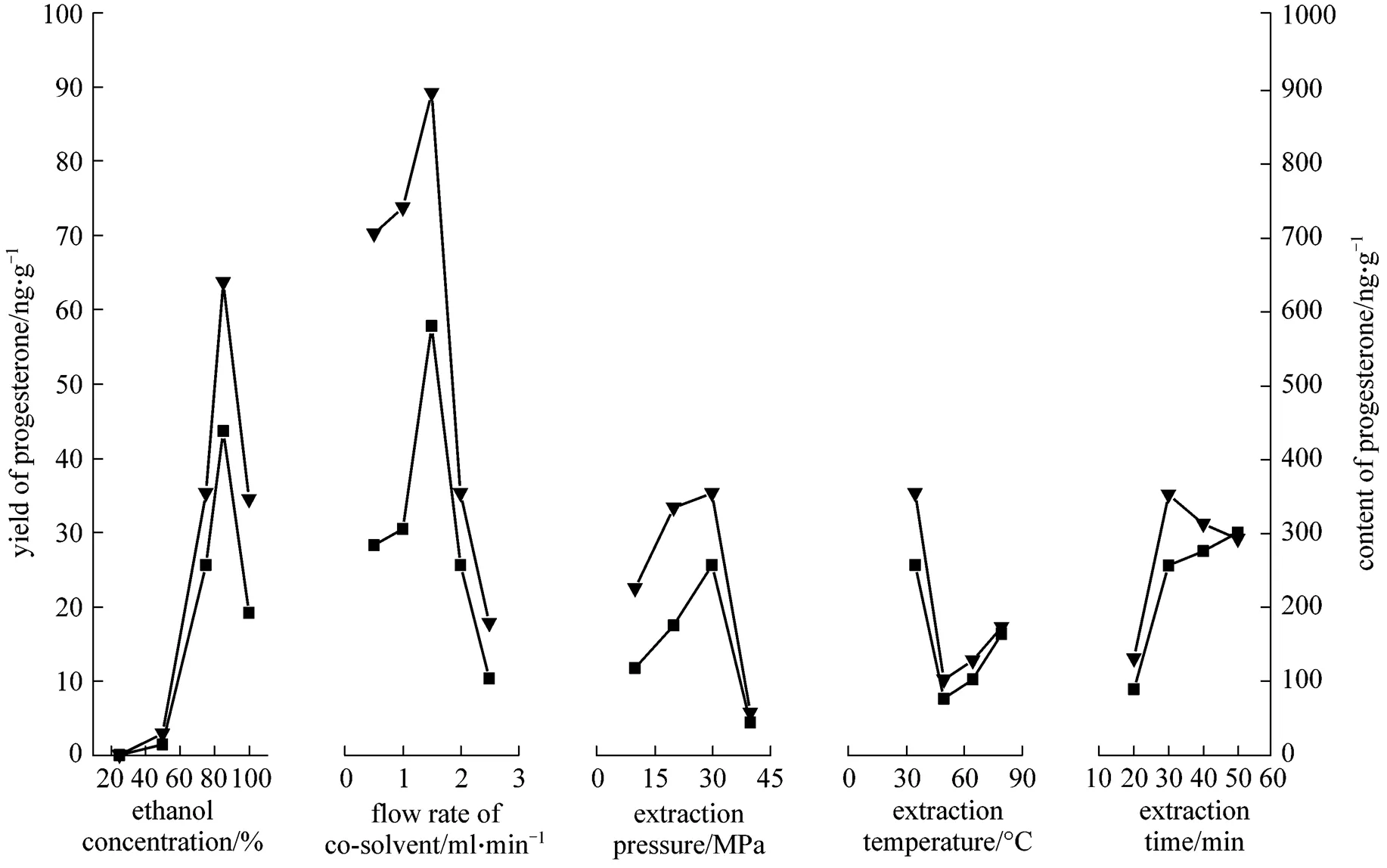
Figure 3 Effect of SC-CO2extraction conditions on yield and content of progesterone■?yield;▼?content
The influences of temperature (35, 50, 65, and 80°C) on the extraction are shown in Figs. 3 and 4. The results indicate that a lower temperature is beneficial for the extraction of estradiol and progesterone. At 35°C, the yield and content of both estradiol and progesterone are the highest.
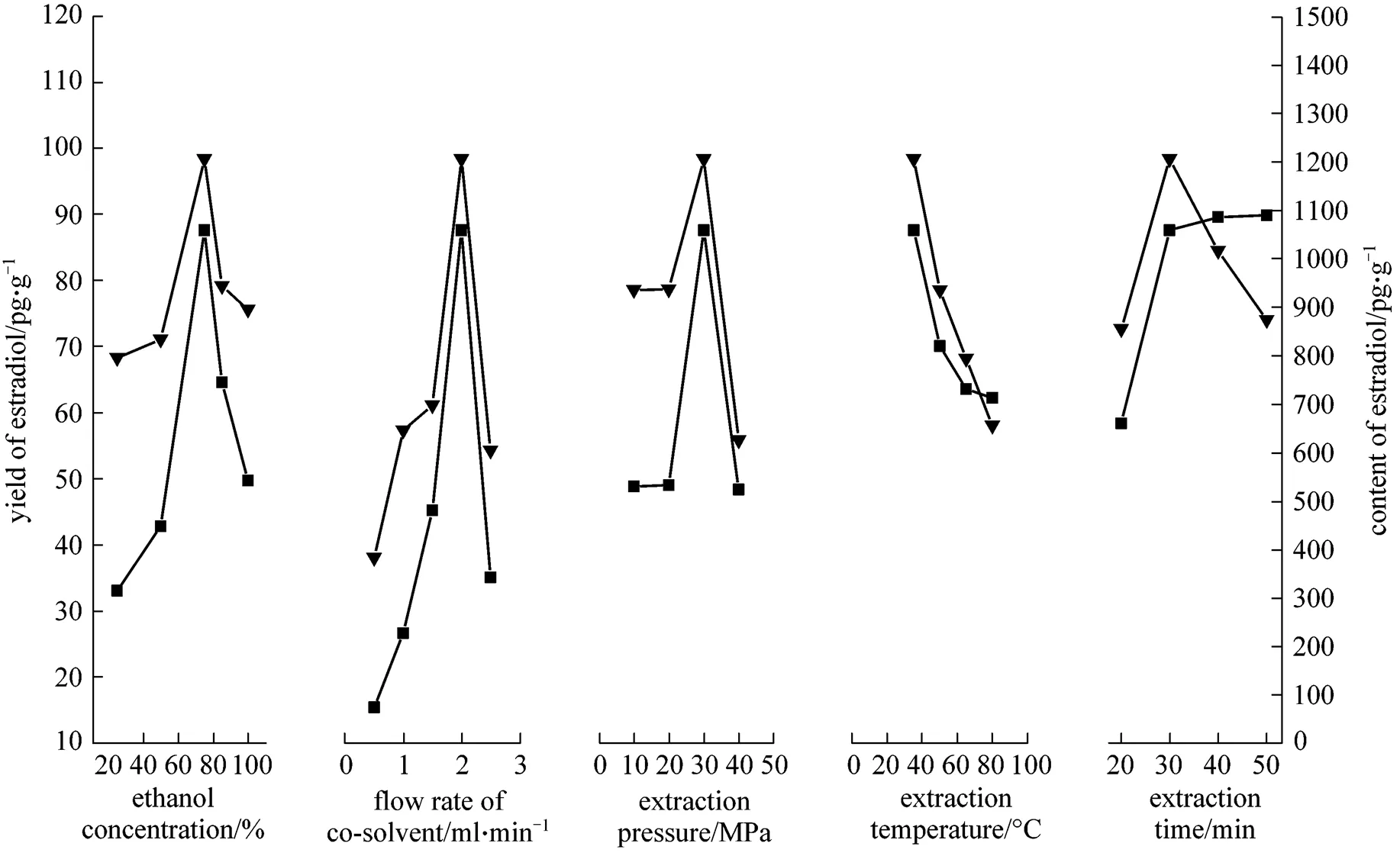
Figure 4 Effect of SC-CO2extraction conditions on yield and content of estradiol ■?yield;▼?content
3.1.4
It can be seen from Figs. 3 and 4 that the yield of estradiol and progesterone increases with the extraction time, and the maximum content of estradiol and progesterone is obtained at 30 min. After 30 min, their yield changes slightly, and the content of them decreases. Therefore, the appropriate extraction time of 30 min is determined.
Finally, the optimal operation parameters for high extraction yield and content of estradiol and progesterone are obtained. When the co-solvent was 75% ethanol, the extraction pressure was 30 MPa, the extraction temperature was 35°C, the extraction time was 30 min, and the consumption of CO2was 15 L·g-1at the flow rate of 2.0 L·min-1, the mean yield and content of estradiol were 87.67 pg·g-1and 1224.10 pg·g-1, respectively, while the yield and content of progesterone were 25.66 ng·g-1and 354.06 ng·g-1, respectively, and the RSD for the four factors were 11.75%, 27.01%, 19.54%, 23.60%, respectively.
3.2 Effect of ultrasonic extraction variables on the content of IGF-1 in antler velvet residue
It is known that IGF-1 is a single-chain polypeptide of 70 amino acids with a high solubility in water solution. Among the factors that affect the content of IGF-1, the following four was investigated: kind of solvent, the ratio of solvent to sample, extraction temperature and time. In all the experiments, the ultrasonicpower was set at 200 W. The content of IGF-1 in antler velvet residue was calculated by the following equation:

As for solvents, different kinds of solvent [sodium acetate buffer solution (pH 3.5, 4.8, 5.8), sodium phosphate buffer solution (pH 6.8, 7.4, 8.9), ammonia- ammonium chloride buffer solution (pH 10)] were tested to extract IGF-1 from antler velvet residue under sonication. The results showed that the mean content of IGF-1 was the highest when using ammonia- ammonium chloride buffer solution with pH10 as the extraction solvent, which was chosen as the best solvent and used in the following extraction experiments.
In general, a larger solvent volume can dissolve constituents more effectively to enhance the extraction yield. The influence of the ratio of solvent to sample onthe content of IGF-1 in antler velvet residue was evaluated.Fig. 5 indicates that the content of IGF-1 increases with the ratio of solvent to sample. When this ratio increases from 18︰1 to 20︰1, the content of IGF-1 changes little. Hence, the ratio of 20︰1 was selected.
The effect of temperature for 15 min extraction time is also shown in Fig. 5. As the temperature increases from 0 to 35°C, there is a slightly decrease of IGF-1 content in the residue. When the temperature increases from 35 to 80°C, the proteins in the residue may degrade and denature, and the content of IGF-1 decreases markedly. Since lower temperature is beneficial for the extraction of IGF-1, 0-35°C was selected.
The influence of extraction time on the extraction content of IGF-1 from antler velvet residue is shown in Fig. 5. The results indicate that the extraction time has little influence on the content of IGF-1 in the residue. It was found that for a total time of 60 min, the content of IGF-1 was the highest when the sample was extracted four times, each for 15 min, using fresh solvent. When the sample was extracted twice and each for 30 min, the yield was higher than that by extraction once for 60 min. Therefore, for a total time of 60 min, the four-step extraction is more suitable to achieve higher content of IGF-1 from dried antler velvet residue by ultrasonic extraction.
Considering the above results, the following experimental conditions were selected as optimum: pH10 buffer solution, the ratio of solvent to sample 20/1 (volume/mass), extraction temperature 0-35°C, and extraction time 4×15 min. The highest content of IGF-1 was up to 7425.75 ng·g-1antler velvet residue.
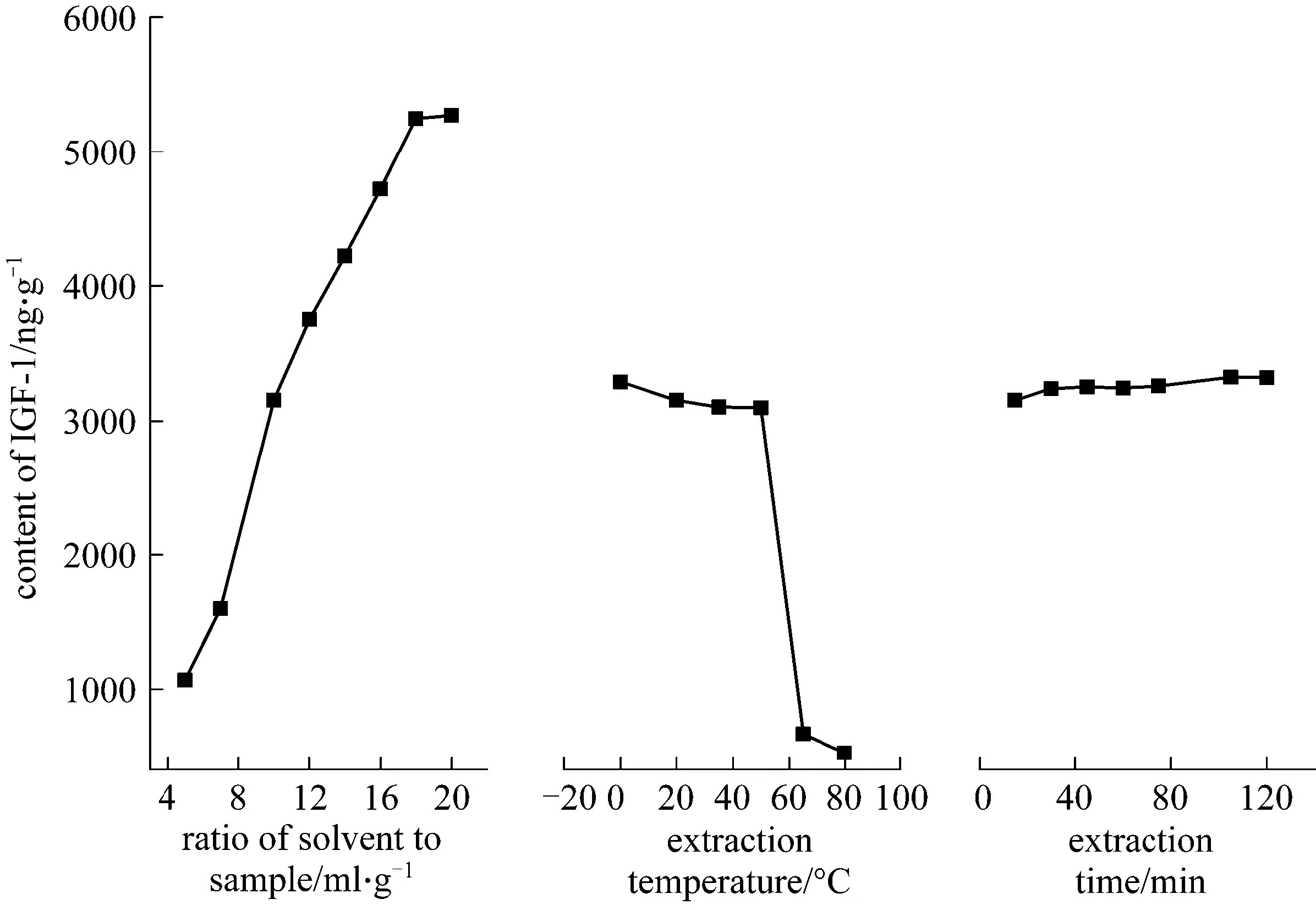
Figure 5 Effect of extraction variables on extraction content of IGF-1
Figure 6 Effect of SC-CO2extraction conditions on activity remaining of IGF-1
3.3 Effect of SC-CO2 extraction variables on the activity remaining of IGF-1
SC-CO2extraction in the presence of dynamic co-solvent was used to extract estradiol and progesterone from antler velvet, and the high yield and content of estradiol and progesterone were obtained under the optimal conditions. In the meantime, because the extraction conditions may have great influence on active IGF-1 in antler velvet, the high activity remaining of IGF-1 in the residue is desired.
3.3.1
Figure 6 shows the effect of ethanol concentration on activity remaining of IGF-1 in the antler velvet residue. As the concentration of ethanol solution increases, the retention of IGF-1 changes slightly. In other words, the activity of IGF-1 is not affected.
3.3.2
The flow rate of co-solvent also has the influence on the retention of IGF-1. On the one hand, with the increase of the flow rate of co-solvent, more contact will make the proteins denatured; on the other hand, for increased contact speed and short contact time, the degree of protein denaturizing may reduce. It is seen from Fig. 6 that when the flow rate of co-solvent is controlled at 2.5 ml·min-1, the remaining activity of IGF-1 is the highest, but it is not favorable to the yield and content of estradiol and progesterone. Therefore, the flow rate of co-solvent from 1.5 to 2.0 ml·min-1was selected in this study after comprehensive analyses of three indexes including the yield and content of both estradiol and progesterone and the retention of IGF-1.
3.3.3
The extraction pressure has some effect on the activity remaining of IGF-1 in the residue. It is seen from Fig. 6 that as the pressure increases from 10 to 30 MPa, the remaining ratio of IGF-1 is slightly improved. At 30 MPa, the activity remaining of IGF-1 in the residue is 93.68%. When the extraction pressure increases to 40 MPa, the activity remaining of IGF-1 in residue is reduced drastically.
The influence of temperatures (35, 50, 65, and 80°C) on the activity remaining of IGF-1 is shown in Fig. 6. The results indicate that a lower temperature is beneficial for keeping IGF-1. Since many water-soluble proteins, glycosaminoglycan, collagen protein and keratin exist in anlter velvet, the proteins may denature and the viscosity of whole system will increase with temperature. When the extraction temperature is 35°C, the retention of IGF-1 is the highest.
3.3.4
The extraction time is associated with the contact time between the co-solvent and material. Longer extraction time and more residual organic solvent will result in a higher degree of degeneration of the active components. It is seen from Fig. 6 that when the time is going on, the remaining ratio of IGF-1 decreases. At 30 min, the activity remaining of IGF-1 in the residue is 93.68%. Therefore, appropriate extraction time of 30 min is determined.
Considering all the results, the following experimental conditions were selected as optimum: When the co-solvent was 75% ethanol, the extraction pressure was 30 MPa, the extraction temperature was 35°C, the extraction time was 30 min, and the consumption of CO2was 15 L·g-1at the flow rate of 2.0 L·min-1, the mean yield and content of estradiol were 87.67 pg·g-1and 1224.10 pg·g-1, respectively, while the yield and content of progesterone were 25.66 ng·g-1and 354.06 ng·g-1, respectively. The activity remaining of IGF-1 was up to 93.68%. The highest content of IGF-1 was up to 7425.75 ng·g-1antler velvet residue.
3.4 Comparison of two sex hormones and IGF-1 by different extraction methods
It must be noticed from the extraction data (Table 1) that estradiol yield (87.67 pg·g-1) and progesterone yield (25.66 ng·g-1) obtained in this study were higher, compared with the yield obtained by refluxing extraction (58.08 pg·g-1and 12.38 ng·g-1; Table 1). It is more interesting that there is a large increase of estradiol and progesterone content, from 832.64 pg·g-1and 169.84 ng·g-1in the refluxing extract to 1224.10 pg·g-1and 354.06 ng·g-1in the SC-CO2and co-solvent extract.
The activity remaining of IGF-1 in the residue treated by this study and traditional extraction method was compared. The activity remaining of IGF-1 in the residue was up to 93.68% under the optimum SFE conditions, while all the IGF-1 was damaged in the traditional residue.
4 Conclusions
SC-CO2and co-solvent extraction followed by ultrasonic extraction is a technically feasible method for extracting estradiol and progesterone from antler velvet and protecting IGF-1 in its residue. This new approach has the characteristic of less consumption of CO2and time, and more effective utilization of active components. In presence of 75% ethanol as the co-solvent, the yield and content of sex hormones were much higher than those by the traditional solvent extraction, with extraction pressure of 30 MPa, temperature of 35°C, extraction time of 30 min and CO2consumption of 15 L·g-1at the flow rate of 2.0 L·min-1.The highest content of IGF-1 was up to 7425.75 ng·g-1antler velvet residue, when the pH10 ammonia- ammonium chloride buffer solution was used as the solvent, the ratio of solvent to sample was 20/1 (volume mass), extraction temperature was 0-35°C, extraction time was 4×15 min. Under these conditions, 93.68% activity remaining of IGF-1 in the residue was obtained, while all the IGF-1 was lost after traditional extraction. The results show the obvious advantage of the combination between SC-CO2and co-solvent extraction and ultrasonic extraction, which can not be achieved with traditional method. The active components can be mostly remained by the new method, which will help to achieve the goal to comprehensively utilize fresh antler velvet.
AcknowledgEmentS
.
1 Kim, K.H., Kim, K.S., Choi, B.J., Chung, K.H., Chang, Y.C., Lee, S.D., Park, K.K., Kim, H.M., Kim, C.H., “Anti-bone resorption activity of deer antler aqua-acupunture, the pilose antler ofTEMMINCK var.(Nokyong) in adjuvant-induced arthritic rats”,.., 96, 497-506 (2005).
2 Li, C.W., Jiang, Z.G., Jiang, G.H., Fang, J.M., “Seasonal changes of reproductive behavior and fecal steroid concentrations in Père David’s deer”,, 40, 518-525 (2001).
3 Sunwoo, H.H., Nakano, T., Hudson, R.J., Sim, J.S., “Isolation, characterization and localization of glycosaminoglycans in growing antlers of wapiti ()”,:, 120, 273-283 (1998).
4 Sunwoo, H.H., Nakano, T., Sim, J.S., “Isolation and characterization of proteoglycans from growing antlers of wapiti ()”,:, 121, 437-442 (1998).
5 Ditchkoff, S.S., Spicer, L.J., Masters, R.E., Lochmiller, R.L., “Concentrations of insulin-like growth factor-I in adult male white-tailed deer (): Associations with serum testosterone, morphometrics and age during and after the breeding season”,:, 129, 887-895 (2001).
6 Barling, P.M., Lai, A.K.W., Nicholson, L.F.B., “Distribution of EGF and its receptor in growing red deer antler”,, 29, 229-236 (2005).
7 Bhavnani, B.R., “Pharmacology of hormonal therapeutic agents”, The Menopause Comprehensive Management, The Parthenon Publishing Group, New York, 229-256 (2000).
8 Froesch, E.R., Schmid, C., Schmander, J., Zapf, J., “Actions of insulin-like growth factors”,..., 47, 443-467 (1985).
9 Rechler, M.M., Nissley, S.P., “The nature and regulation of the receptors of insulin-like growth factors”,..., 47, 425-442 (1985).
10 Jones, J.I., Clemmons, D.R., “Insulin-like growth factors and their binding proteins: Biological actions”,.., 16, 3-34 (1995).
11 Lowe, W.L.Jr., “Biological actions of the insulin-like growth factors”, Insulin-Like Growth Factors: Molecular and Cellular Aspects, CRC Press, Boca Raton FL, 49-85 (1991).
12 Daughaday, W.H., Rotwein, P., “Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum and tissue concentrations”,, 10, 68-91 (1989).
13 Ling, Y., Luo, F.C., Li, Q.X., “Assay of estradiol and testosterone in pilose antler”,, 30, 499-501 (1999). (in Chinese)
14 Yang, R.M., Zhang, J.H., Cao, H., Zhang, L.Y., Bao, T.N., Zhao, S., Ding, X.H., “Determination of sex hormones in milu antler by HPLC analysis”,..., 29, 618 (2001). (in Chinese)
15 Li, C.W., Jiang, Z.G., Zeng, Y., Yan, C.E., Zhang, L.Y., Xia, J.S., Tang, B.T., “Velvet tissue estradiol concentrations in Père david’s deer, sika deer and fallow deer”,, 49, 124-127 (2003).
16 Moore, L.G., Jones, D., Lymburn, M.A., Hodgkinson, S.C., Davis, S.R., Suttie, J.M., Sagighi, M., Carne, A., “Isolation and sequencing of deer and sheep insulin-like growth factors I and II”,, 92, 302-310 (1991).
17 Mason, T.J., Cordemans, E.D., “Ultrasonic intensification of chemical processing and related operations: A review”,...., 74, 511-516 (1996).
18 Schinor, E.C., Salvador, M.J., Turatti, I.C.C., Zucchi, O.L.A.D., Dias, D.A., “Comparison of classical and ultrasound-assisted extractions of steroids and triterpenoids from three Chresta spp”,.., 11, 415-421 (2004).
19 Li, H., Chen, B., Yao, S.Z., “Application of ultrasonic technique for extracting chlorogenic acid from. (.)”,.. 12, 295-300 (2005).
20 Jacques, R.A., dos Santos Freitas, L., Pérez, V.F., Dariva, C., de Oliveira, A.P., de Oliveira, J.V., Caram?o, E.B., “The use of ultrasound in the extraction of Ilex paraguariensis leaves: A comparison with maceration”,.., 14, 6-12 (2007).
21 Rodrigues, S., Pinto, G.A.S., Fernandes, F.A.N., “Optimization of ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder by response surface methodology”,.., 15, 95-100 (2008).
22 Posyniak, A., Zmudzki, J., Semeniuk, S., “Effects of the matrix and sample preparation on the determination of fluoroquinolone residue in animal tissues”,..., 914, 89-94 (2001).
23 Hardcastle, J.L., Paterson, C.J., Compton, R.G., “Biphasic sonoelectroanalysis: Simultaneous extraction from, and determination of vanillin in food flavoring”,, 13, 899-905 (2001).
2008-09-11,
2009-02-17.
the Natural Science Foundation of Tianjin (06YFJMJC10500).
** To whom correspondence should be addressed. E-mail: shfli@tju.edu.cn
 Chinese Journal of Chemical Engineering2009年3期
Chinese Journal of Chemical Engineering2009年3期
- Chinese Journal of Chemical Engineering的其它文章
- Position Group Contribution Method for Estimation of Melting Point of Organic Compounds
- Process Intensification of VOC Removal from High Viscous Media by Rotating Packed Bed*
- Adsorption of Dye from Wastewater by Zeolites Synthesized from Fly Ash: Kinetic and Equilibrium Studies*
- Modeling of Isomerization of C8 Aromatics by Online Least Squares Support Vector Machine*
- Resolution of Ibuprofen Ester by Catalytic Antibodies in Water-miscible Organic-solvents*
- Reaction Characteristics of Asymmetric Synthesis of (2S,5S)-2,5-Hexanediol Catalyzed with Baker’s Yeast Number 6*
